|
Mr. Rogers' AP Physics C: IB Physics Topics |
||||
| Syllabus | 1st Quarter | 2nd Quarter | 3rd Quarter | 4th Quarter |
| IB SL Thermo | IB HL Thermo | IB HL Waves | AP Review | |
Topic 9: HL Thermal Physics (SL optional) - Thermodynamic Processes
| 1st Law | 2nd Law | Processes | Heat Engines | HL Equations |
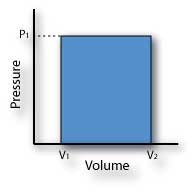
An isobaric process requires a large amount of heat input. The process is a suitable model for the fuel injection portion of a Diesel cycle.
W = ∫ P(v) ∙ dv or area under the curve
W = P1 (v2 - v1)
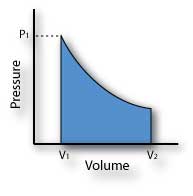
An isothermal process requires a moderate amount of heat input. This process is found in the idealized Carnot cycle.
For a perfect gas:
Pv = nRT
P = nRT/v
W = ∫ P(v) ∙ dv
= ∫ nRT/v ∙ dv integrate from v1 to v2
W = nRT ln|v2/v1|

The compression and expansion cycles of internal combustion, piston-driven engines occur so fast that they are considered adiabatic. Essentially there is not enough time for heat transfer.
Since no heat is transferred in, adiabatic expansions do less work than an isothermal expansion with the same starting conditions and ending volume.
An isochoric process cannot do work since there is no change in volume.
