| Qh = | the heat flow into the heat pump from a high temperature (Th) reservoir. | |
| Qc = | the heat flow from the heat pump into a low temperature (Tc ) reservoir. | |
| W = | The heat pump's work output | |
- From the 1st Law of Thermo:
- Qh = Qc+ W
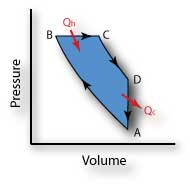
Carnot Cycle - the perfect heat engine
| 1. | Isothermal expansion A → B: | |
| Qh = in, W = out, T↔, P↓, V↑ | ||
| 2. | Adiabatic expansion B → C: | |
| Qh = 0, W = out, T↓, P↓, V↑ | ||
| 3. | Isothermal compression C → D: | |
| Qc = out, W = in, T↔, P↑, V↓ | ||
| 4. | Adiabatic compression D → A: | |
| Qc = out, W = in, T↑, P↑, V↓ |

Otto Cycle - typical gasoline engine
| 1. | Intake stroke O → A: the piston moves down drawing air and fuel into the cylinder. | |
| Q = 0, W = 0, T↔, P↔ V↑ | ||
| 2. | Adiabatic compression A → B: the piston moves upward. | |
| Q = 0, W = in, T↑, P↑, V↓ | ||
| 3. | Isochoric combustion B → C: the spark plug fires and the gasoline/air mixture burns. | |
| Qh = in, W = 0, T↑, P↑, V↔ | ||
| 4. | Adiabatic expansion C → D: the piston moves downward in the power stroke. | |
| Q = 0, W = out, T↓, P↓, V↑ | ||
| 5. | Isochoric pressure drop D → A: the exhaust valve opens dropping the cylinder pressure. | |
| Qc = out, W = 0, T↓, P↓, V↔ | ||
| 6. | Exhaust stroke A →O: the piston moves upward pushing the exhaust products out of the cylinder. | |
| Q = 0, W = 0, T↔, P↔, V↓ |

| 1. | Adiabatic compression A → B: | |
| Q = 0, W = in, T↑, P↑, V↓ | ||
| 2. | Isobaric expansion B → C: Fuel injection occurs during this part of the cycle | |
| Qh = in, W = out, T↑, P↔, V↑ | ||
| 3. | Adiabatic expansion C → D: | |
| Q = 0, W = out, T↓, P↓, V↑ | ||
| 4. | Isochoric pressure drop D → A: | |
| Qc = out, W = 0, T↓, P↓, V↔ | ||
Heat Pumps transfer heat from a cold to a hot region or backwards with respect to normal heat flow. To do so requires a work input. Heat pumps will typically transfer more heat than the work input required to run them and are an economical form of heating for buildings in areas with mild winters. In this operation they transfer heat from cold outside air into warm inside air.
Air conditioners and refrigerators are also heat pumps but transfer heat from cool inside air to warm outside air.
Like heat engines, heat pumps also have thermodynamic cycles but run in reverse. The most efficient would be a reversed Carnot cycle run in reverse.
- From the 1st Law of Thermo:
- Qh = Qc+ W
- COP(heating) = Qh /W
- Note: Qh = Qc + W
- Here the work done to run the HP actually shows up as useful heat.
- From Carnot cycle:
- COPmax(heating) = Th /(Th - Tc)
- COP(cooling) = Qc /W
- Note: Qc = Qh - W
- Here the work done to run the HP is wasted.
- From Carnot cycle:
- COPmax(cooling) = Tc /(Th - Tc)

